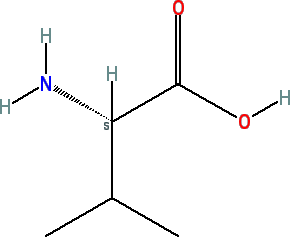
Valine
Safety Information
U.S. Food and Drug Administration (FDA)
The U.S. Food and Drug Administration (FDA) lists the amino acids, including their hydrochloride (HCl), sodium and potassium salts as food additives permitted to be directly added to food. The FDA permits glycine to be used in over-the-counter (OTC) antacid drug products.
Expert Panel for Cosmetic Ingredient Safety
The safety of the alpha amino acids and their simple salts has been assessed by the Expert Panel for Cosmetic Ingredient Safety. The Expert Panel evaluated the scientific data and concluded that these ingredients were safe as used in cosmetics and personal care products.
The normal presence of these ingredients in the body and their use as direct food additives led the Expert Panel to focus their review only on dermal irritation and sensitization data. Dermal data on products containing these ingredients indicated that the amino acids are not dermal irritants or sensitizers. The Expert Panel noted that some individuals have issues with dietary sodium glutamate and phenylalanine. The Expert Panel determined that the concentrations of these amino acids used in cosmetic products are lower than levels that would result in significant exposure.
Based on the available data, the Expert Panel concluded that the amino acids and their simple salts are safe for use in cosmetic products.
FDA: Link to Code of Federal Regulations for the amino acids.
The amino acids and their simple salts may be used in cosmetics and personal care products marketed in Europe according to the general provisions of the Cosmetics Regulation of the European Union.