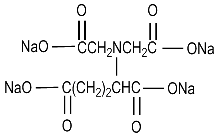
Tetrasodium Glutamate Diacetate
Safety Information
Expert Panel for Cosmetic Ingredient Safety
The safety of tetrasodium glutamate diacetate was assessed by the Expert Panel for Cosmetic Ingredient Safety (formerly called the Cosmetic Ingredient Review Expert Panel) in 2021. The Expert Panel concluded that tetrasodium glutamate diacetate was safe as used in the present practices of use and concentration in cosmetics and personal care products.
The Expert Panel found that the systemic toxicity data – including reproductive toxicity and fetal developmental studies, acute and sub-chronic repeated dose toxicity studies, and skin irritation and sensitization studies – were sufficient for assessing safety for reported cosmetic uses of tetrasodium glutamate diacetate.
The Expert Panel previously reviewed the safety of additional α-amino acids, including alanine, glutamic acid and sodium glutamate, and concluded that these ingredients are safe in the present practices of use and concentration in cosmetics.
The Expert Panel noted tetrasodium glutamate diacetate is slowly absorbed through the gastrointestinal tract and skin absorption is likely to be even slower. The Expert Panel also noted the lack of carcinogenicity (cancer-causing potential) data and that tetrasodium glutamate diacetate may contain a salt of nitrilotriacetic acid, a category 2B carcinogen according to the International Agency for Research on Cancer (IARC). However, the concern was mitigated by multiple genotoxicity studies (both in vitro and in vivo), which did not indicate adverse genetic activity at the low concentrations of this ingredient in leave-on products.
Tetrasodium glutamate diacetate is listed in the EU’s Cosmetic Ingredient Database (CosIng) and may be used safely under the rules governing cosmetic products in the Cosmetics Regulation of the European Union and used as a chelating agent in cosmetics.